Potential Concerns for Tree Wound Response from Stem Injection
Kevin T. Smith1 and Phillip A. Lewis2
1USDA Forest Service, Northeastern Research Station,
271 Mast Road, Durham, NH 03824
2USDA Animal and Plant Health Inspection Service,
Pest Survey, Detection and Exclusion Laboratory,
Building 1398, Otis ANGB, MA 02542
ABSTRACT
Stem injection of imidacloprid is an available component of management strategies for hemlock woolly adelgid. Preliminary observations of similar treatments of maple and ash show that the injury sustained by injection warrants investigation of the wound response in eastern hemlock. Such investigations need adequate experimental controls to identify the role of phytoxicity of the active ingredient and the carrier formulation, delivery pressure, seasonality, and tree condition. External indicators such as bark cracks tend to underestimate the amount of cambial dieback. Evaluation of the wound response requires tree dissection. We suggest that the unintended consequences of treatment such as injection injury be considered and incorporated into the management decision process.
KEYWORDS
Stem injection, tree injection, compartmentalization, imidacloprid.
INTRODUCTION
Hemlock woolly adelgid (HWA), Adelges tsugae Annand, is an aphid-like insect pest that can kill mature eastern hemlock, Tsuga canadense (L.) Carr., trees within a few years of infestation. HWA is one of a growing list of introduced insect pests that threaten wild, managed, and urban forests in the eastern United States. Because of the widespread occurrence and importance of eastern hemlock, diverse strategies to manage HWA are being tested, including pest exclusion, roguing of infested individuals, biological control through the release of predators and parasitoids, and the application of chemical insecticides. Although some combination of cultural practices and biological control may eventually protect susceptible forests from HWA, chemical insecticides will likely continue to be used over the next years to reduce HWA severity and spread.
The direct injection of chemical treatments into trees through stem injection has a long history. In recent years, concern over spray drift and the runoff from sprays and soil applications of insecticides has renewed attention to stem injection. Imidacloprid (a chloro-nicotinyl insecticide) in various formulations is the primary insecticide being injected to control HWA and other introduced, invasive pests. Stem injections are part of tests or control strategies for HWA in Pennsylvania, Massachusetts, and Connecticut. The appeal of stem injection lies in the expectation of the delivery of effective doses into targeted tissues (such as branchlets in the case of HWA) throughout the tree translocation system with few or no nontarget effects and a high degree of social acceptance. That appeal, coupled to the potential commercial profitability of the technique suggests that stem injection will continue to be considered as a treatment option for trees infested or threatened by HWA.
The biological tradeoff with stem injection is that the tree is wounded in the course of treatment (Smith 1988). Although the size of the mechanical wound made during injection may be small, the treatment chemical and the application pressures can greatly increase the severity of wound-initiated discoloration and cambial dieback associated with an injection (Shigo et al. 1977).
Tests of efficacy of chemical formulation and injection methods generally focus on the recovery of the treatment chemical or its metabolites from the targeted plant part with some attention given to comparisons of infestation intensity and growth recovery. Rarely do tests of efficacy of injected pesticides include evaluation of the effects of tree injury and wound response, particularly for recently introduced injection technology.
Should the wound response of hemlock injected with imidacloprid or other insecticides be investigated? No critical dissection studies of injected eastern hemlock stems have been published at this time. However, observations of injected and dissected red maple (Acer rubrum L.), sugar maple (Acer saccharum Marsh.), and white ash (Fraxinus americana L.) may focus attention on what should be looked for in future field trials of various injection techniques in hemlock.
METHODS
Sugar maple and white ash dissected for this study were part of a larger trial of the systemic distribution of imidacloprid administered by stem injection at the Mount Greylock State Reservation in Lanesborough, Massachusetts. Trees selected for injection in 2002 were 30-40 cm DBH and growing along the access roadway at the reserve. Injections were made by professional applicators experienced in the methods used. Although the trees were vigorous and apparently healthy, they were likely under moisture stress at the time of injection, making them less than ideal sample trees for testing. Trees were injected in mid August while following the manufacturer recommendations in effect at the time using one of two stem injection methods. The two methods represent two different types of injection schemes. For both systems, injection sites were usually located at the trunk flare, 10-30 cm above ground line. With the Arborjet Viper System (Arborjet Inc., Winchester, Massachusetts), each injection site was prepared by drilling an 8-mm-diameter hole along the stem radius to a depth of about 4 cm into the sapwood. The hole was snugly fitted with a proprietary brass injection port (a prototype of the current plastic Arborplug). Injection sites were distributed along a spiral around the circumference of the lower stem. The number of injection sites was calculated as one-half the stem diameter at DBH, in inches. A proprietary 5% imidacloprid solution was used with an average of 10 to 14 mls of formulation injected through the ports at a delivery pressure of ranging from 300-600 PSI.
Also tested was the Wedgle Direct-Inject System (ArborSystems, Omaha, Nebraska). The Wedgle injection site was prepared by removing a 2-mm-diameter plug to a depth of about 4 mm in the bark, using a special tool. The hole did not extend to the vascular cambium or the wood. The hole was fitted with a hard plastic injection port filled with silicon (the Wedgechek). Injection sites were prepared for every 6 inches of trunk circumference. Two proprietary imidacloprid formulations were used to inject the trees: 12 and 20% ‘Pointer’. Two ml of insecticide was attempted at each injection site at an unknown delivery pressure.
In September 2003, the injected trees were examined externally, and two sugar maple and two white ash injected by each of the two treatments were felled and bucked. The bolts containing the injection sites were taken to a workshop for examination and further dissection through bark removal, sawing, and splitting.
OBSERVATIONS
After one or two growing seasons, external cracks in the bark were associated with some of the Arborjet and a few of the Wedgle injection sites. When present, the vertical cracks passed through the Arborjet injection sites and 1-2 cm to the side of the Wedgle injection sites (Figure 1).
Stem dissection indicated that the visible extent of external cracks greatly underestimated the amount of cambial dieback, particularly for Wedgle injection sites (Figure 2). Even when there were no cracks evident from the outside of the bark (Figure 2A), the vascular cambium and phloem were killed as seen on the inside of the bark (Figure 2B) and from the wood surface of the dissected tree (Figure 2C) In vigorously growing trees, callus was produced at the margins of the cambial dieback and the cell derivatives had initiated woundwood formation (Figure 2C). Imidacloprid residue around both Wedgle and Arborjet and injection sites was commonly found (Figs. 2B, C and 3).
Internal columns of wound-initiated discoloration were generally well-defined for Arborjet injection sites for both ash and maple (Figure 3). Little wound-initiated discoloration was evident in the Wedgle injection sites.
DISCUSSION
Because of the small sample size, these observations must not be construed to support a preference for one injection technique over another. Similarly, no differences in tolerance between the tree species can be assessed. Because of the short time period between injection and dissection, the long-term effect of single or repeated rounds of injection treatments also cannot be assessed. Although the possible moisture stress at the time of injection may have hindered treatment uptake, the degree of stress was likely well-within the range of what is expected for urban and community trees.
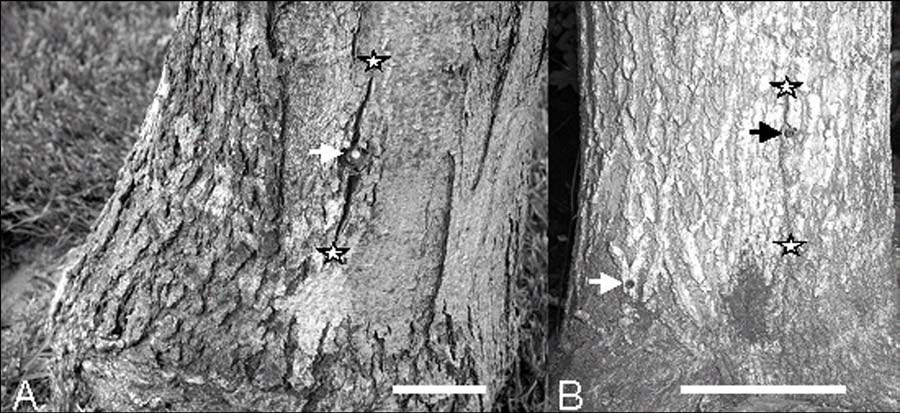
Figure 1. Bark cracks associated with stem injection. The injection sites (arrows) and the vertical limits of the cracks (stars) are marked. (A) Arborjet injection of sugar maple. (B) Wedgle injection of white ash. Scale bars = 5 cm.
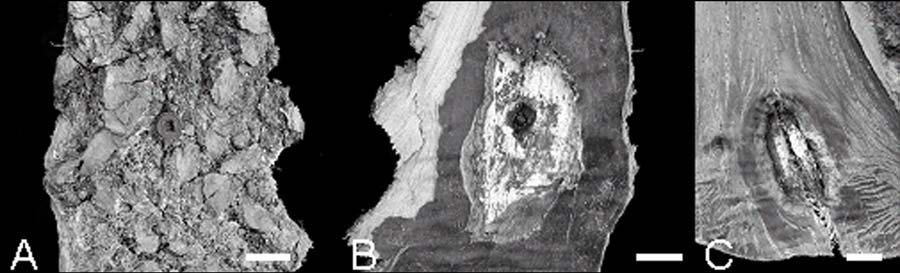
Figure 2.Stem injury from Wedgle injection in dissected white ash. (A) External view of injection site with “arborcheck” plug. (B) Reverse surface of (A) showing plug, killed inner bark, and imidacloprid residue. (C) Stem surface beneath the bark showing the area of cambial dieback, woundwood formation, and imidacloprid residue. Scale bars = 5 mm.

Figure 3. Stem injury from Arborjet injection in dissected sugar maple. Note the wound initiated discoloration and the imidacloprid residue. Scale bar = 10 cm.
We observed that the injection treatments cause a greater amount of injury than is evident from external examination. Dissection studies are needed to assess the extent of cambial dieback and wound-initiated discoloration associated with the injection site. Cambial dieback is a more accurate indicator of the size of the injury than the size of the mechanical injector or external cracking. Given sufficient time in otherwise vigorous trees, woundwood produced at the margins of the cambial dieback will likely close over the wound. Longerterm research is needed to determine if closure results in the desired restoration of cambial continuity around the stem circumference or whether the ribs or rolls of woundwood will press against each other to form stem cracks.
Dead bark and phloem were located to the outside of the killed vascular cambium. To the inside were sapwood cells killed directly by the treatment or indirectly by desiccation and disconnection from living phloem. Compartmentalization is the boundary-setting process that resists the spread of cell death and subsequent infection by microorganisms in wounded wood and bark (Shigo 1984). Frequently, the killed sapwood within compartmentalization boundaries is a different color than healthy sapwood and is referred to as wound-initiated discoloration. More important than the change in wood color is that the formerly alive cells in healthy wood now are dead in discolored wood and incapable of (1) energy storage, (2) active shifts to defensive physiology, and (3) water conduction.
Effective compartmentalization minimizes the volume and resists the spread of woundinitiated discoloration associated with an injury. The Wedgle system induced little woundinitiated discoloration after one growing season. The extensive cambial dieback associated with the Wedgle system is at least in part due to the intentional separation of bark from underlying tissues that is designed to form a reservoir for the injected chemical. Tests using longer intervals of time between injection and dissection are needed to determine if woundinitiated discoloration will develop beneath the cambial dieback.
For these observations, it is impossible to assess whether the injury was due to imidacloprid or the components of the injection method. In addition to longer incubation times, future tests of the wound response from chemical injections need adequate experimental controls. At least until the role of these factors have been well-documented, the simple and interactive effects of the size and type of injector, the delivery pressure, the active ingredient, the vehicle formulation, tree condition, and seasonality of injection should all be tested.
It can be argued that these observations, although recent, no longer reflect the latest technology. Indeed, over the past decades injection technology has swung from using high pressure to low pressure to passive infusion and back again. Formulations have employed high concentrations of active ingredients at low volumes and dilute concentrations at high volumes. New modifications are continually in development. These changes can aid practical management but should not be used as an excuse to avoid critical testing for the effects of injection.
The imidacloprid residue associated with both injection methods needs further examination. Is the presence of residue due to less-than-ideal conditions for uptake of the chemical treatment or is it inherent in the employed techniques? Should the presence of this residue be considered as a nontarget release of the chemical into the environment?
How might these findings be different for eastern hemlock? Previous studies indicate that the tracheid system in conifers can require higher injection pressures for treatment uptake (Sanchez-Zamora and Fernandez-Escobar 2004 ), causing greater injury. Compartmentalization in conifers usually involves enhanced biosynthesis of terpenes and resin production, which can complicate the assessment of effectiveness of compartmentalization.
We suggest that stem injection as well as other measures to control or manage HWA be considered using a treatment matrix that contains the overlapping dimensions of efficacy, consequences of inaction, the frequency of repeated treatments, direct economic cost, availability of alternative treatments, social acceptance, and unintended consequences of treatments to include the tree wound response.
ACKNOWLEDGEMENTS
We thank Kenneth A. Gooch (Massachusetts Bureau of Forestry, Pittsfield, Massachusetts) for his assistance with the tree dissections.
REFERENCES
Shigo, A.L. 1984. Compartmentalization: a conceptual framework for understanding how trees grow and defend themselves. Annual Review of Phytopathology 22:189-214.
Shigo, A.L. W.E. Money, and D. Dodds. 1977. Some internal effects of Mauget tree injections. Journal of Arboriculture 3:213-220.
Sanchez-Zamora, M.A. and R. Fernandez-Escobar. 2004. Uptake and distribution of trunk injections in conifers. Journal of Arboriculture 30:73-79.
Smith, K.T. 1988. Wounding, compartmentalization, and treatment tradeoffs. Journal of Arboriculture 14:226-229.
